Alkalinity In Boiler Water
Monitoring alkalinity in boiler water is essential to ensure system efficiency and safety. When levels fall outside of safe operating ranges, issues like corrosion, embrittlement, and carryover can occur—especially in cooling water systems, where chemical treatments for scale and corrosion become less effective.
The Three Basic Forms of Alkalinity
Alkalinity in water appears in three chemical forms depending on pH:
- Carbonate (CO₃)
- Bicarbonate (HCO₃)
- Hydroxide (OH⁻)
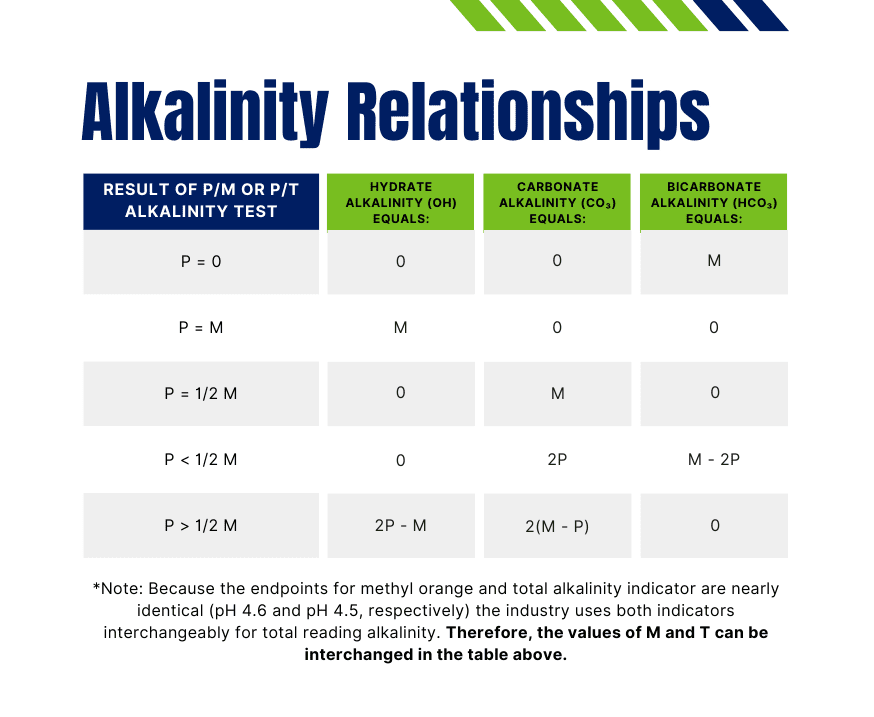
Total alkalinity is the sum of these three and is measured by titrating a water sample with a standard acid. Results are expressed as P, M, or T alkalinity:
- P Alkalinity: Titrated to pH 8.3 with phenolphthalein
- M Alkalinity: Titrated to pH 4.6 with methyl orange
- T Alkalinity: Titrated to pH 4.5 using a total alkalinity indicator
You can use the table of relationships below to determine treatment control and effectiveness.
How is Alkalinity Maintained?
Treatment specialists work to keep M (or T) alkalinity below the maximum limits recommended by the boiler manufacturer. Higher boiler pressures require higher water purity. At the same time, OH⁻ alkalinity (generally above 150 ppm as CaCO₃) is necessary to prevent scale formation and maintain overall chemical stability.
In cooling water, proper alkalinity management is critical:
Too low → corrosion
Too high → calcium scaling
For systems that need better control, pH treatment solutions like pHREADY can help stabilize water chemistry and maintain alkalinity levels.
Alkalinity Testing Interference
When managing alkalinity titrations, highly turbid or colored samples will mask the color change at the endpoint. Chlorine may also interfere with the sample as well, so we recommend sodium thiosulfate to counteract any interference. Suspended solids can be filtered out of the sample using paper filtration, and colloidal colors may require syringe filtration. Treatment polymers will titrate as M and T alkalinity. In waters using polymer treatment, OH alkalinity needs to be directly titrated using the barium chloride method. Using a 2P-M method to determine OH alkalinity will result in a negative interference for the calculation of OH. Absorption of CO2 will depress alkalinity in water samples. To prevent this situation, analyze samples at the point of collection. When on-site analysis is not practical, collect samples for off-site testing in overflowing containers and cap them tightly. If acid is being added to adjust pH, it will also alter the P/T alkalinity relationship. If you notice foul smells during your testing, our pHREADY for Odor Control can help you treat the issue at the source while balancing the alkalinity in your system.
About The Author
Nick Piskura is the Marketing and Web Development Specialist at ChemREADY who utilizes expertise in digital marketing strategies to provide knowledgeable insights in each segment of our business. Nick provides insights through web development and multimedia resources that support ChemREADY’s full range of services, including Legionella management, ANSI/AAMI ST108 compliance, boiler and cooling tower treatment, wastewater processing, and industrial water quality solutions.

