Boiler Water Treatment Maintenance BMP’s
Why is Boiler Water Treatment Maintenance Required?
No matter the type of boiler you work with, corrosion is always a risk and not everyone understands the preventative boiler maintenance, or water boiler treatment chemicals program, required to prevent equipment damage.
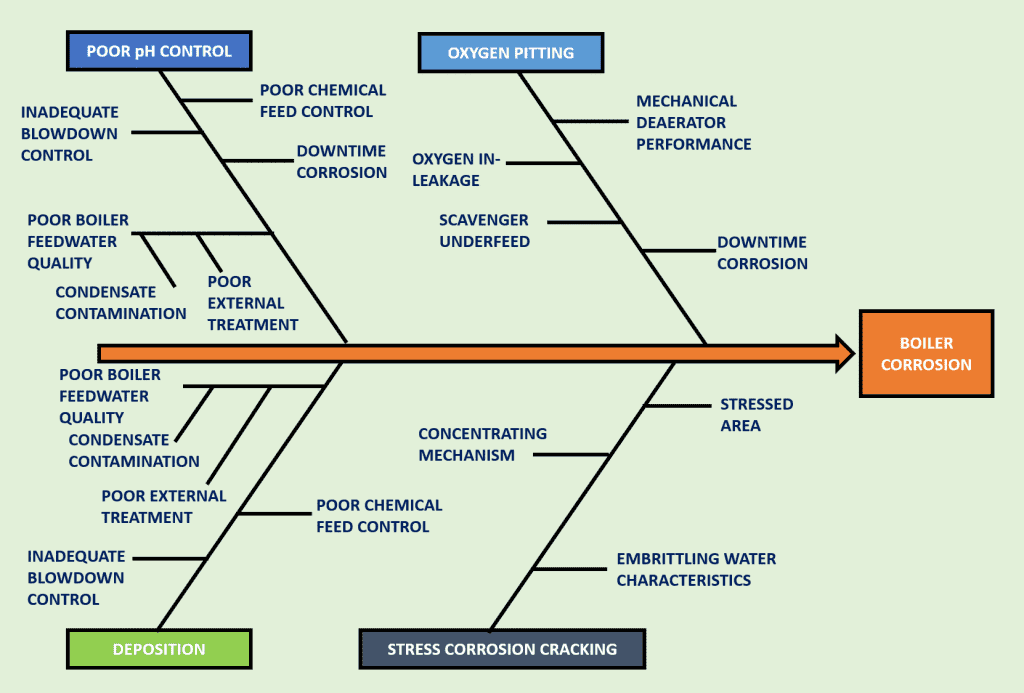
Corrosion is commonly caused by oxygen or improper pH control. This can create holes in economizers, boiler tubes or feedwater piping resulting in boiler leaks and a pricey fix (see the next section for more). There are many forms of corrosion and they are not treated equally.
It is necessary to consider the quantity of the various harmful substances that can be allowed in the boiler water without the risk of damage to the boiler. Corrosion may occur in the feed-water system as a result of low pH water and the presence of dissolved oxygen and carbon dioxide.
Corrosion can be minimized through proper design (to minimize erosion), periodic cleaning, and constant and consistent control of oxygen, pH, and dissolved solids. So when you ask yourself, why is boiler water treatment chemicals required, the best method of ensuring peak performance level is through continuous control and utilizing an automated chemical feed and monitoring system to ensure the use of high-quality feedwater (and promote passivation of metal surfaces). Deaerators are also used to heat feedwater and reduce oxygen and other dissolved gases to acceptable levels in some facilities as an additional means of preventative treatment to the automated feedwater systems and can help to reduce the amount of chemical consumed.
ChemREADY is a boiler water treatment company specializing in boiler water treatment maintenance. Contact us to learn more.

Major monitoring parameters required for water treatment:
- Dissolved solids
- pH of the boiler feed water
- Dissolved Oxygen in the feed water entering the boiler
- Silica in boiler water
Automated feed systems provide the following benefits to any preventative maintenance water treatment program:
- Little to no handling of chemicals for employees (less chance of chemical spills)
- No overdosing of chemicals (potentially costing more money)
- No under-dosing of chemicals (allowing potential corrosion to occur)
- Ability to monitor and track system consistency (both operations and cost related)
How Much Does Boiler Maintenance Cost?
Because of a boiler’s vital function for any facility, their breakdown can result in safety concerns, not to mention a huge cost in order to replace or repair the system. Repair costs to boilers can be steep and can range anywhere from a few thousand dollars to over $1 million depending on size, function and accessibility. But it doesn’t stop there. This price is in addition to the expense of operational down times to get the boilers repaired or replaced and up and running properly.
The cost of automating a boiler water feed system can range anywhere from a few thousand dollars up to $50K, depending on the size, demand and number of boilers any one facility may have in place.
What Are The Disadvantages Of Boiler Corrosion?
Minutes’ worth of imbalance in a water treatment program can cause problems, therefore, the fewer fluctuations in a water treatment program and water quality feeding the boiler the better it is for the equipment. When a system is not continuously treated and tested for accuracy, chemical imbalances can occur, allowing minutes, hours or days’ worth of potential corrosion and scaling to occur.
The graph below is known as the Baylis Curve. It shows the relationship between pH, alkalinity, and water stability. Water above the lines is scale-forming while water below the lines is corrosive. Stable water is found in the white area between the lines.
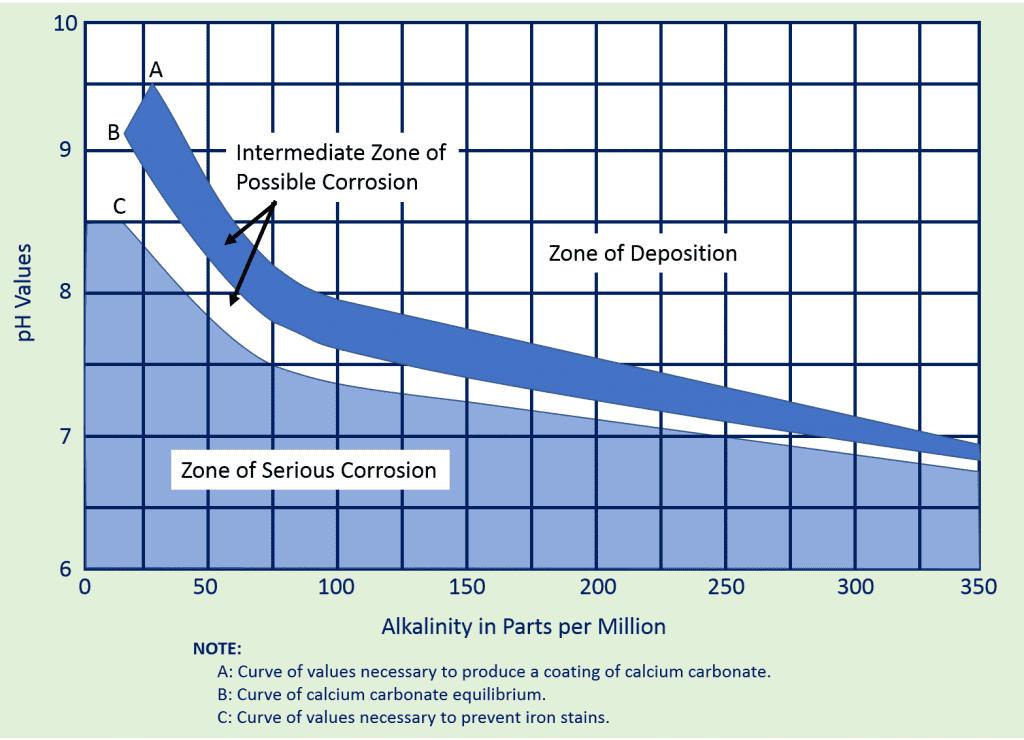
As you can see by the above graph, there is a fairly “fine line” between corrosive and not corrosive when it comes to a heated boiler water system. The pH, alkalinity, temperature of the water and various other factors will play a role in dictating whether or not there is a possibility that the water has gone corrosive and has begun to eat away at your infrastructure.
In these scenarios, we like to refer to these systems as “not if’s but when’s”, because we know it is only a matter of WHEN the corrosion will be enough to cause a problem and not a matter of IF it will.
Keep in mind that corrosion is the only SYMPTOM and the CAUSE is inconsistent feed water.
Why Is Water Quality Important?
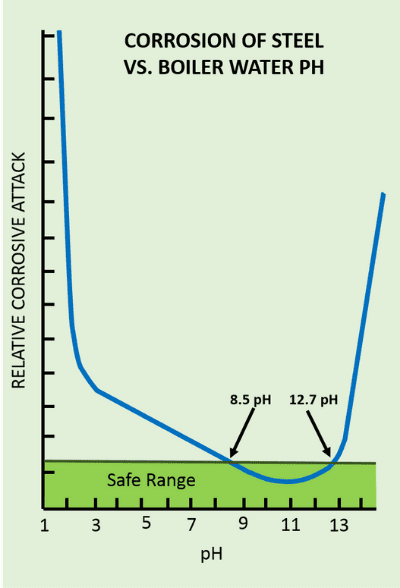
Water contains dissolved salts, which upon evaporation of water forms scales on the heat transfer surfaces. Scales have much lower heat transfer capacity than steel: the heat transfer coefficient of the scales is 1 kcal/m/°C/hr against 15 kcal/m/°C/hr for steel. This leads to overheating and failure of the boiler tubes. Scale also reduces flow area, which increases pressure drop in boiler tubes and piping.
Low pH or dissolved oxygen in the water attacks the steel. This causes pitting or lowering the thickness of the steel tubes, leading to rupture of the boiler tubes. Contaminants like chlorides, a problem in seawater cooled power plants, also behave in a similar way.
Flow assisted corrosion occurs in the carbon steel pipes due to the continuous removal of the protective oxide layer at high flows.
Impurities carried over in the steam, causing deposits on turbine blades leading to reduced turbine efficiency, high vibrations, and blade failure. These contaminants can also cause erosion of turbine blades. Silica at higher operating pressures volatilizes and carries over to the turbine blades.
What Maintenance Does A Boiler Need?
1. The first step is to get the make-up water to the steam cycle as pure as possible.
2. The second step is to form a protective layer on the inside surface of the tubes which protects the metal surface from any further corrosion attacks.
3. The third step is to maintain this layer throughout the life of the plant. If the water quality goes down, this protective layer will be destroyed and corrosion starts damaging the tubes.
How Do You Maintain A Boiler System?
Even the most aggressive forms of prevention can’t stop minor corrosion from eventually happening. But, with the right approach, the effects of corrosion can be minimized and extend the life of your boiler. While ChemREADY cannot reverse time and corrosion – we can certainly stop corrosion in its tracks!
Here’s what to do to minimize the effect of corrosion before it happens:
Use a boiler logbook. Regularly tracking the normal operation of your boiler room equipment makes it easy to spot when something critical changes.\
- Deaerator pressure or feed-tank temperature changes will give advance warning of a more expensive corrosion problem. pH changes could indicate problems with water treatment or process contamination.
Treat Feedwater. Additives can ensure that any oxygen that makes its way to the boiler in the feedwater is rapidly absorbed before it has the opportunity to form corrosive cells and blisters.
- Work with a good water chemistry company (like ChemREADY!) to stay on top of your boiler water.
Implement a strict, regular service program to ensure the boiler stays clean and free of scale and corrosion problems. Train employees to ensure a complete understanding of the boiler system, how it operates and its importance
- If the boiler is being inspected, the root cause can be addressed early, avoiding more costly repairs.
For hydronic systems, check for leaks and monitor the quantity of make-up water. Hot water heating systems shouldn’t need make-up water unless something is wrong. Call your service provider to fix the leak right away, or you may be replacing the boiler next year.
Automate boiler chemical feed and surface blowdown to maintain uniform chemical residuals and conductivity levels.
How To Treat Corrosion Damage?
- Make necessary repairs to boiler and piping (such as having boiler re-tubed)
- Train your crew on boiler preventative maintenance and water chemistry tests
- Document and report any signs of corrosion to your boiler service provider and your water chemical company so they can help prevent further damage.
Use our tips to ensure the longevity of your boiler. Need some expert advice or repair services? Contact ChemREADY today to schedule your free consultation.
Did You Know?
What Are Different Types Of Corrosion?
Caustic Corrosion. When a concentrated caustic substance dissolves the protective magnetite layer of a boiler. This is commonly caused by boiler water pH being too high, steam blanketing (poor circulation) or local ‘film boiling’. If your boiler has a porous scale, then under deposit corrosion is also possible. Boiler water pH should be a part of your logbook.
Acidic Corrosion. Results from the mishandling of chemicals during acid cleaning operations or the boiler pH being run too low to passivate the carbon steel surfaces of the boiler. Boiler water pH should be a part of your logbook.
Pitting Corrosion. This is one of the most destructive types of corrosion, as it can be hard to predict before a leak forms. Pitting is a localized form of corrosion, in which either a local anodic point or more commonly a cathodic point, forms a small corrosion cell within the surrounding normal surface. Oxygen in feedwater is a common cause of boiler tube pitting. If your boiler is pitting, investigate the proper operation of your deaerator or feedwater tank and chemical treatment. If you have a hot water system, oxygen pitting can occur if the system has a leak and is bringing in fresh water.
Crevice Corrosion. This type of corrosion is also a localized form of corrosion and usually results from a crack in the boiler that does not get good circulation to rinse away caustic.
Galvanic Corrosion. Galvanic corrosion is the degradation of one metal near a joint or juncture that occurs when two electrochemically dissimilar metals are in electrical contact in an electrolytic environment. So, dissimilar metals may need a special dielectric joint, sacrificial anode, or active cathodic protection system to prevent this phenomenon.
About The Author
Nick Piskura is the Marketing and Web Development Specialist at ChemREADY who utilizes expertise in digital marketing strategies to provide knowledgeable insights in each segment of our business. Nick provides insights through web development and multimedia resources that support ChemREADY’s full range of services, including Legionella management, ANSI/AAMI ST108 compliance, boiler and cooling tower treatment, wastewater processing, and industrial water quality solutions.