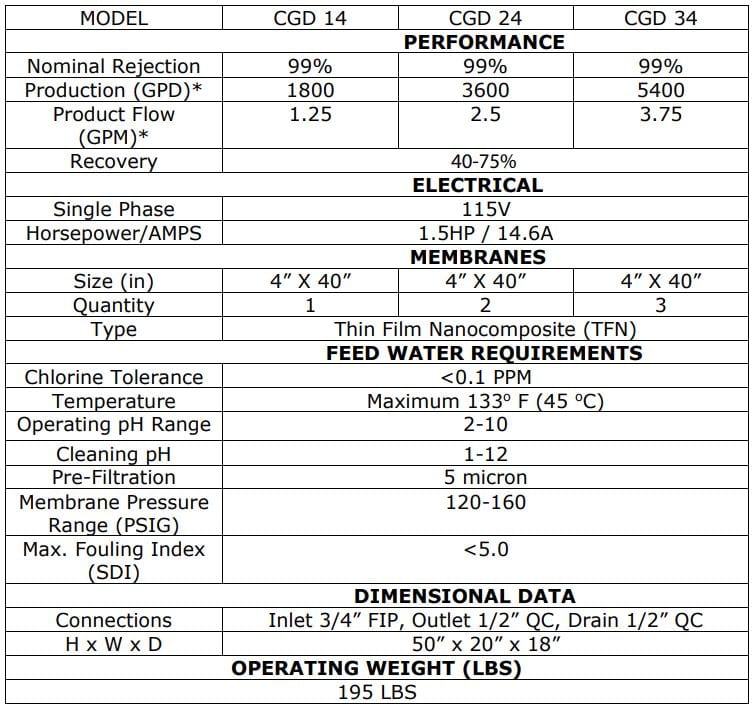
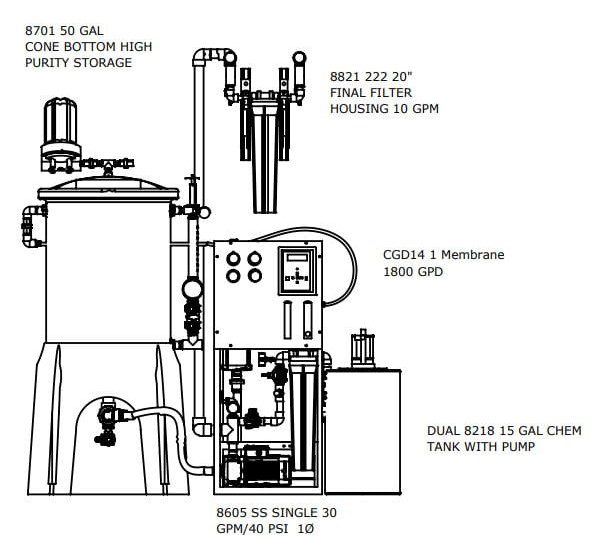
The Central Sterile Water Systems are designed to meet or exceed the proposed standards
The ANSI/AAMI ST108 standard, also known as the “Water for the processing of medical devices” standard, is a comprehensive set of guidelines for the design, construction, and operation of Central Sterile Departments (CSDs). The standard outlines the minimum requirements for water quality and steam purity necessary to effectively process medical devices intended for use on a patient. This standard was developed by the Association for the Advancement of Medical Instrumentation (AAMI) and is recognized as the gold standard for CSD operations.
The ANSI/AAMI ST108 standard is important because it helps to ensure that medical devices are sterilized to the highest standards, minimizing the risk of patient infections. Contaminated medical devices can transmit a variety of pathogens, including bacteria, viruses, and fungi, which can lead to serious infections in patients. By adhering to the AAMI ST108 standard, healthcare facilities can help to prevent these infections and protect the safety of their patients.
AAMI ST108 will not allow for direct feed systems in central sterile processing any longer, and thank goodness! The loop velocity of 3-5 feet per second being recirculated continuously through a 0.2 final filter will ensure that the last water that touches surgical instruments is free of bacteria and endotoxins. This means less surgical site infections!!
The technical information report 34 (TIR34) converted to Standard ST108 and covers the selection and maintenance of effective water quality suitable for reprocessing medical devices in central sterile water facilities. It provides guidelines for selecting the water quality necessary for the reprocessing of categories of medical devices and addresses water treatment equipment, water distribution and storage, quality control procedures for monitoring water quality, strategies for bacterial control, and environmental and personnel considerations.
Water can be treated by a variety of methods that yield different levels of water quality. In general, as the chemical quality of water improves, its microbial content could increase unless the system is closely monitored to prevent microbial overgrowth. Gram-negative bacteria and nontuberculous mycobacteria can grow in any type of water, including tap, softened, deionized (DI), reverse osmosis (RO) treated, and distilled water. The rate of growth and the microbial levels attained are a function of the amount of organic contaminants in the water. The importance of monitoring water quality to prevent problems with microbial overgrowth cannot be overemphasized.
The AAMI ST108 Central Sterile Water Systems are designed to meet or exceed the proposed standards put forth. The improved process water used in Water Systems lowers infection, protects equipment and improves patient outcomes.
The best practice design provides for larger flow RO with a smaller fresh storage for your Critical Water Delivery System. The design is compliant with AAMI TIR34 and brass/copper/lead free with rust free powder coated aluminum frame. Our deluxe controller with conductivity and optional facilities management communications package make it the most advanced solution on the market.