Surgical site infections are a leading cause of hospital re-admissions, many times due to water quality. Most sterilizers, washers and disinfectors used in the cleaning of surgical instruments are considered medical devices and require treated water within standards set by AAMI and IFU’s of the various device manufacturers. This Guide provides information on:
Water is the universal solvent with the ability to dissolve almost everything it encounters. The properties of water are constantly changing. Wherever it flows, it takes along chemicals, minerals, and nutrients whether it be from the air, the ground and even our bodies.
Although municipalities remove these contaminants to meet US Drinking Water Standards, water becomes re-contaminated as it travels to the final point of use. Additional treatment may be needed based on the specifications for use.
Water that has been treated to be suitable for drinking is not necessarily suitable for Sterile Processing. This is especially true during the final rinse cycle where high quality / low endotoxin water is needed.
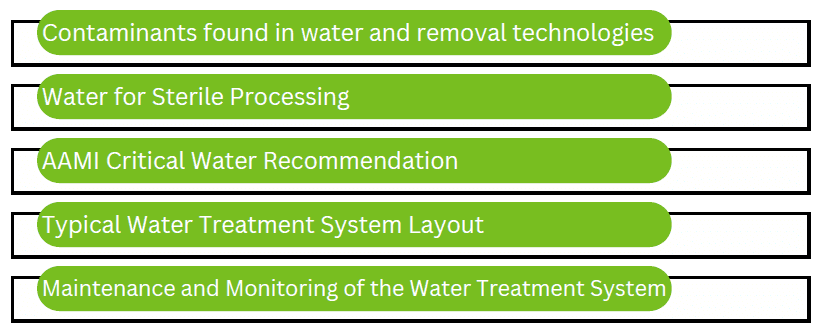
Treated water is for both instrument processing, and final flushing at the sink. The manufacturers of the processing equipment (sterilizers, washers, etc.) each have their own set of specifications and instructions for use (IFU) specifying the quality and quantity of water required for their specific piece of equipment. See Reference Table 1 for a list of equipment and the general water quality specifications.
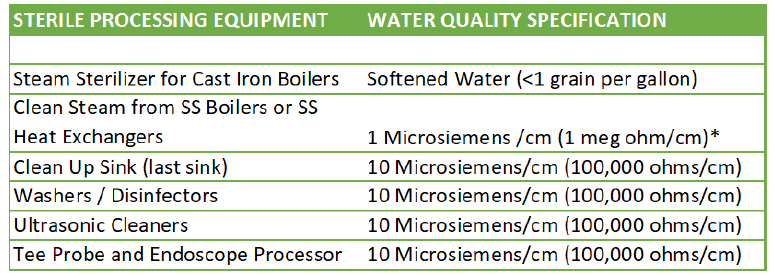
A water treatment company will use the manufacturers specifications to properly size the correct system based on quality and usage. To guarantee no down time, the water treatment system should be sized to supply all the sterile processing devices for one hour. This can be determined by multiplying the water usage for each cycle by the number of cycles per hour by the quantity of devices in the department. Sizing this way guarantees there will be enough water whether the department is running 10 or 24 hours a day and will minimize the size of the pure water storage tank.
What does this mean for you? TIR is a technical information report to be used as a guideline. After several years, this guideline has become a standard that will be audited and monitored by regulatory agencies.
This leads to many questions:
Let’s take this one step at a time.
No doubt, there will be many helpful articles, webinars, and consultants offering information on the new standards. However, it is strongly encouraged that all facilities purchase and study the new written standard from AAMI.
Website: AAMI.org
Name of Standard: AAMI ST108:2023 – Water for Processing of Medical Devices
Compare the new standards against your current system. It’s not just about the equipment you have, but also the maintenance and monitoring. Depending on your time and capabilities, it may be beneficial to bring in an expert who knows the new standard. A few suggestions of who to contact would be:
The new standard includes the requirement of having a Water Management Program. This team is responsible for ensuring all requirements are put in place with continued monitoring and maintenance. A successful team would include an executive who has the authority to allocate resources, facilities engineering personnel, infection prevention and control personnel, medical device processing personnel, clinical engineering personnel, surgical procedures personnel, and a water treatment specialist.
There are several areas in the new standard that suggests you pay special attention to:
Categories of medical devices and the risk levels associated with each:
The difference between Utility Water, Critical Water and Steam
The need for a Water Management Program
How water quality affects the SPD process
This document does not provide details on the standard but can be used as guidance to help you understand where to begin.
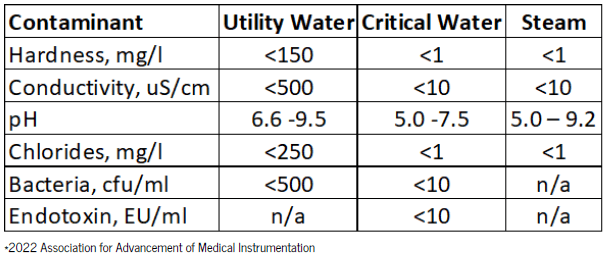
There will be three categories of water quality that may require treatment:
1. Utility Water
Utility Water is the hospitals water source coming straight from the tap. Since this water is used only for flushing, washing, and rinsing the acceptable contaminant levels are less stringent than for Critical Water, but still may require treatment.
2. Critical Water
Critical Water is used for the final rinse on the washer/disinfector and steam generation. For the health and safety of the patient, it’s important to remove microorganisms such as bacteria and endotoxin.
3. Steam
Steam is vaporized water that is produced by a boiler or heat exchanger. The condensate testing should meet the requirements as shown in table 2.
This list is not comprehensive and shows only an overview of the main contaminants.
For the full list refer to AAMI ST108.
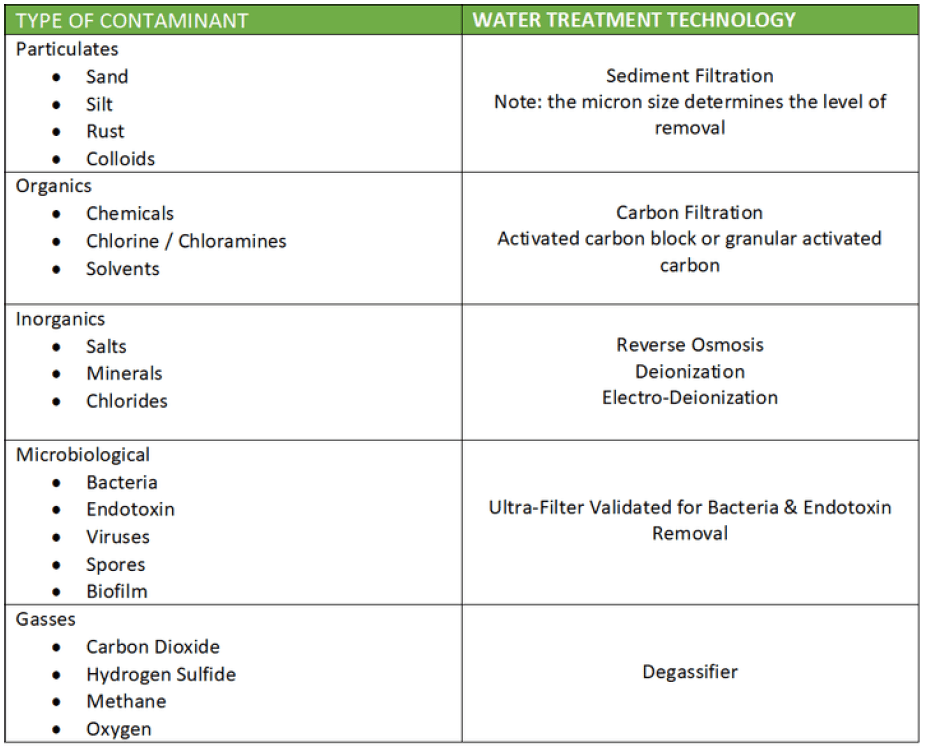
Contaminant Control Technology ANSI AAMI ST108
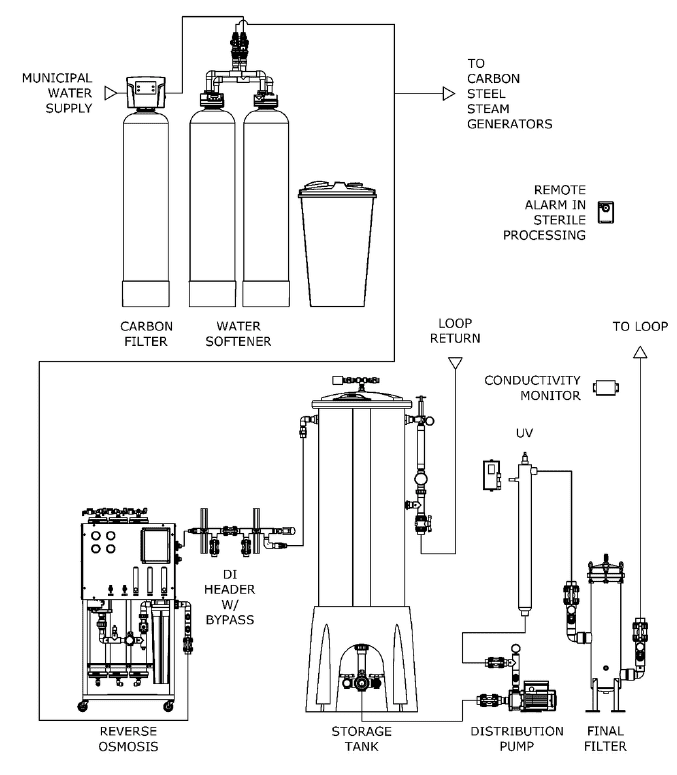
Typical ANSI AAMI ST108 Layout for Critical Water
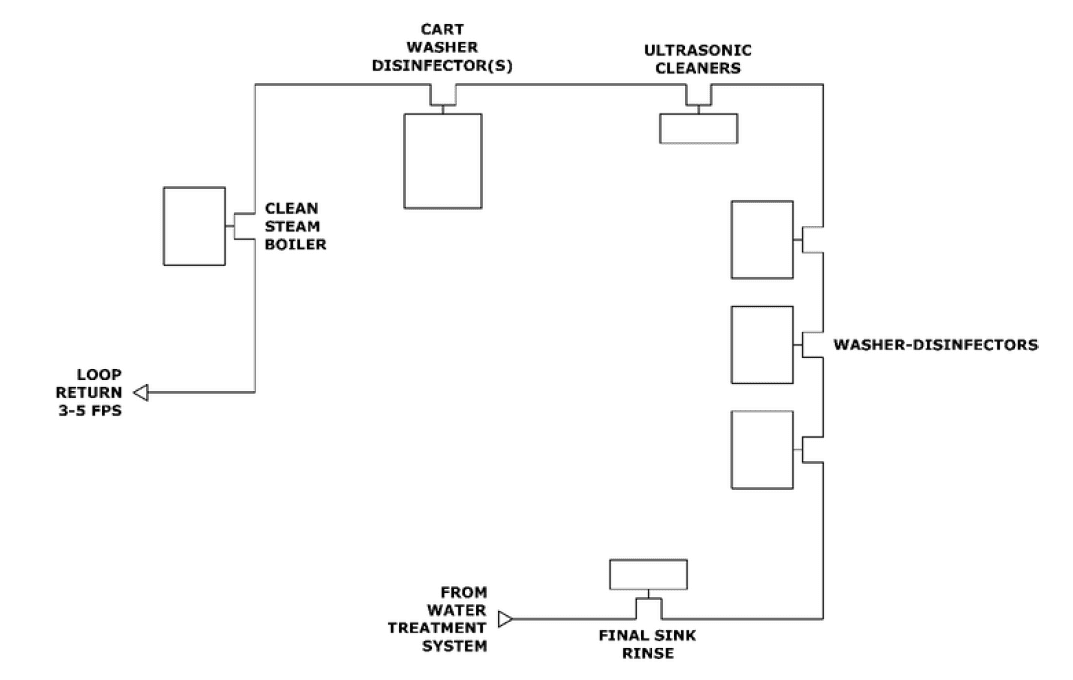
Sterile Processing ANSI AAMI ST108 Layout
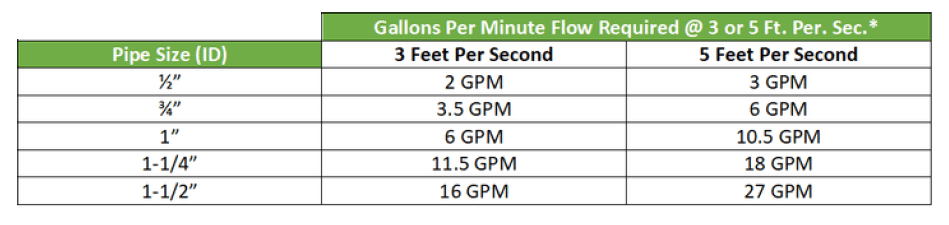
High purity water needs to be distributed in high quality plastic or stainless-steel piping to prevent contamination. Recirculation and prevention of dead legs is important for regular disinfection of the distribution system. To prevent biofilm in the piping and connections, distribution loops should recirculate at a continuous rate of 3-5 feet per second and the pipe feeding the device should be less than six times the pipe diameter of the loop. See chart below for recommended recirculation rates in distribution loop:
*Based on Schedule 80 PVC. Other piping systems may be slightly different.
A critical water treatment system requires monitoring, testing, disinfection, and on-going maintenance including consumable component replacements. Every day the systems are operating, parameters should be logged assuring that the equipment is performing properly.
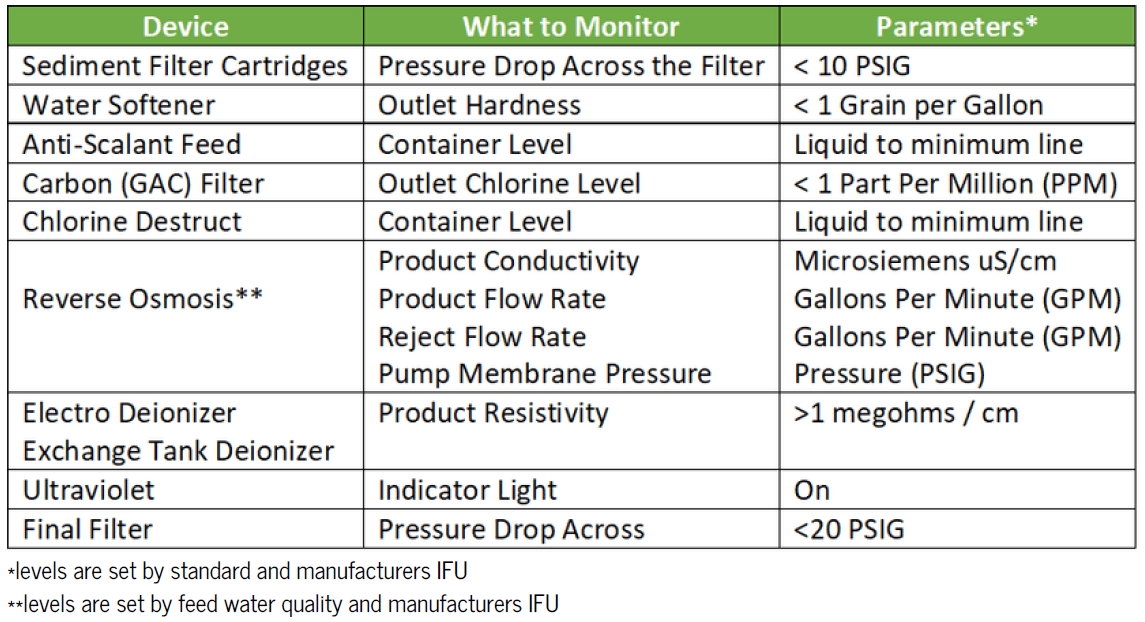
Most likely you are already monitoring the critical water. It will now be necessary to monitor the Utility Water as well since it is included in the new standards. Water sampling should always be taken before disinfection. If results are above level, disinfect and rinse to the level of no detection, then retest. The best disinfectant for reverse osmosis, storage tank and distribution loop is 1% Peracetic Acid or Ozonation.
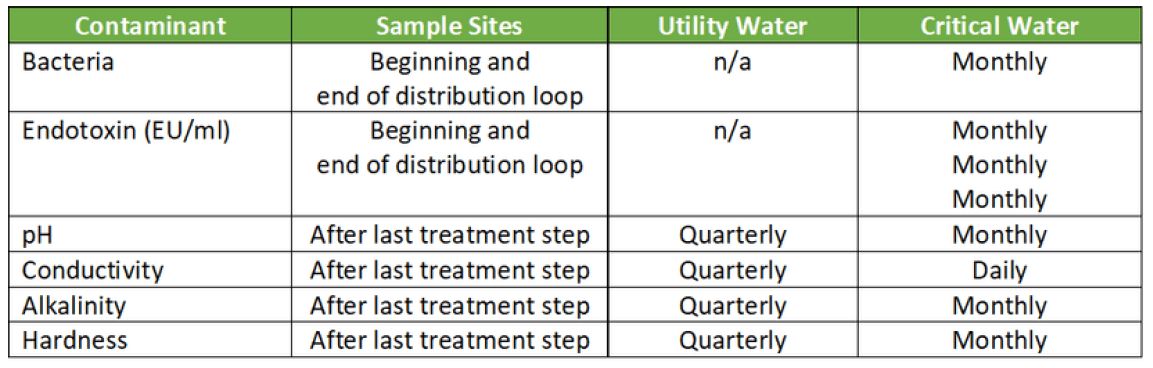
Water quality varies from season to season, place to place, and even day to day.
Because it plays a significant role in the sterilization process, it is important to team up with a knowledgeable water treatment company who specializes in the health care industry and follows the guidelines set by AAMI/ANSI ST108:2023. In addition, and possibly most important, the team of sterile processing professionals must remain informed and vigilant in following all procedures to ensure the safety of their patients.